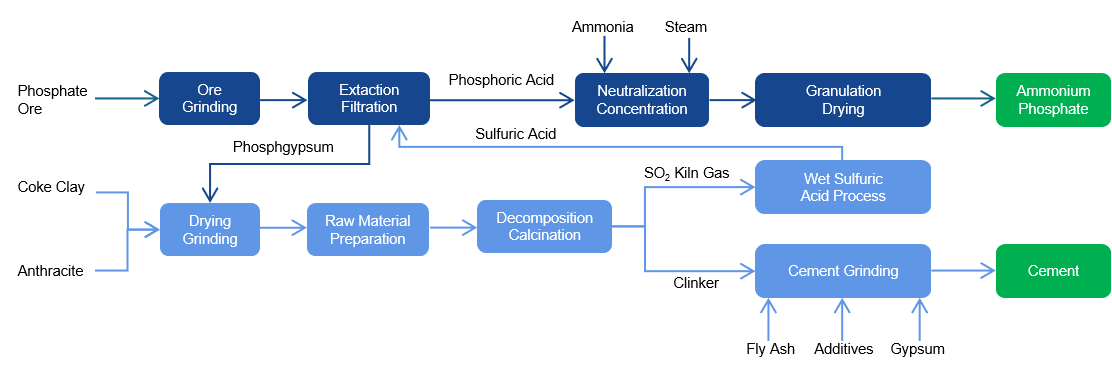
Gypsum Calcination and Decomposition:
CaSO4+2C → CaS+2CO2↑ (900~1000℃ ) (1)
3CaSO4+CaS → 4CaO+4SO2↑ (1000~1200℃ ) (2)
2CaSO4+C → 2CaO+2SO2↑+CO2↑ (1+2)
SO2 flue gas to sulfuric acid (SO2: 4-6%mol, prior with wet process):
SO2+1/2 O2 → SO3 (400~500℃)
SO3+H2O→H2SO4
The CaO derived from the decomposition of gypsum together with the SiO2, Al2O3, Fe2O3 formes the four main mineral components of cement clinker:
12CaO+3SiO2+Al2O3+Fe2O3→CaO·SiO2(CS)+2CaO·SiO2(C2S)+3CaO·SiO2(C3S)+4CaO·Al2O3·Fe2O3(C4AF)
Weight Propotion of Cement P.O.42.5:
CaO: 64-67%, SiO2: 20-23%, Al2O3: 4-8%, Fe2O3: 3-6%
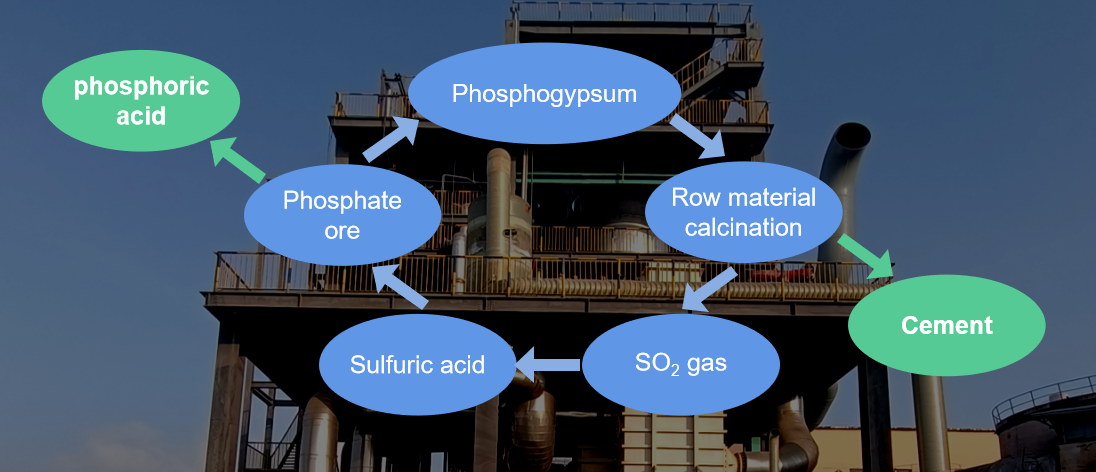
The recycling process can treat phosphogypsum and produce two products, cement and sulfuric acid, and recycle.
Effectively solved the gypsum landfill disposal issues and its consequently environmental problem. Improve the plant as a green, environmental-friend plant and optimize the social-responsibility of end-user.
15ys experience with phosphogypsum decomposition and recycling.
Economic benefit, the product (cement and sulfuric acid) benefit is higher than the operation cost.

Owner : LuBei Phosphate Chemcals Co., Ltd.
Location : East part of China
Plant in operation : 15+years
Operation plant : 2250T/D phosphogypsum cracking and recycling 1000T/D sulfuric acid and 1250T/D cement.
Feedstock : Phosphogypsum
Prodcution: Sulfuric acid 98%wt routed back to wet phosphoric acid plant; P.O.42.5 cement product as sales.